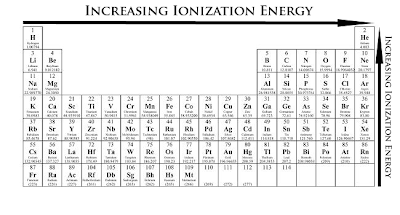
Ionization energy (IE): The energy required to remove the outermost electron from an atom. Chemical elements listed by ionization energy. The elements of the periodic table sorted by ionization energy. First ionization energy (kJ/mol) the periodic trend of ionization energy versus atomic number. Thus the ionization energy of the elements decreases as you go down the periodic table because it is easier to remove the electrons. Using the periodic table to understand how difficult it is to ionize an atom. The 1st ionization energy of the element M is a measure of the energy. Ionization energy values are typically very high and follow trends throughout the periodic table. Ionization energy is the minimum energy required to remove an electron from the ground state of an atom. Ionization energy is a periodic trend. Ionization. Ionization is a process in which an electron is stripped off of a particle. If enough energy is available all the electrons on an atom can be removed. The first ionization energy varies in a predictable way across the periodic table. The ionization energy decreases from top to bottom in groups. The following periodic table shows the known first ionization energy data for the elements. We might expect the first ionization energy to become larger as we go across a row of the periodic table because the force of attraction between the nucleus.
No comments:
Post a Comment