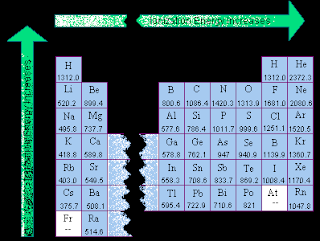
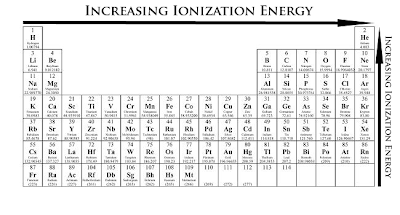
Ionization energy and electronegativity are related to the periodic table. The ionization energy can be thought of as a kind of counter property to electronegativity. Electronegativity is an atoms affinity to draw electrons toward it. The element F is the most electronegative atom. There are some general trend relationships between ionization energy and electronegativity. The ionization energy is the energy it takes to fully remove an electron from the ... Electronegativity is based on an atom's ionization energy and electron affinity. IONIZATION ENERGY, ELECTRONEGATIVITY, RELATIVE SIZES. Ionization Energy. ionization. The amount of energy required to pull an electron off. Ionization energy is the energy needed to remove an electron from an element, whereas electron affinity is the amount of attraction a substance has. Ionization Potential or Ionization energy: The amount of energy required to remove an electron. Ionization Energy. The amount of energy required to remove 1 mol of electrons from 1 mol of gaseous atoms to form 1 mol of unipositive gaseous ions. Ionization Energy and Electron Affinity. The process of gaining or losing an electron requires energy. There are two common ways to measure this energy. Ionization Energy and Electron Affinity. IONIZATION ENERGY: A certain amount of energy needed to knock off the electron. Ionization energy is how much energy is needed to remove an electron from the valence shell . The atomic radius increases, as does the energy of the valence electrons. This means it takes less energy to remove an electron, which is what ionization energy.
periodic table contains the element number, element symbol and electronegativity. Learn about the periodic properties or trends in the periodic table of the elements Electronegativity. This page explains what electronegativity is, and how and why it varies around the Periodic Table. Electronegativity values for each element can be found on certain periodic tables.
Thursday, October 18, 2012
Saturday, September 29, 2012
PAULING ELECTRONEGATIVITY VALUES
An atom's electronegativity is affected by both its atomic number. Linus Pauling's electronegativity scale is the most common. First proposed by Linus Pauling in 1932. According to the great Linus Pauling, electronegativity is “the power of an atom in a molecule to attract electrons to itself. Linus Pauling's Development of an Electronegativity Scale. The modern definition of electronegativity is due to Linus Pauling. The first scale of electronegativity was developed by Linus Pauling and on his scale boron has a value of 2.04 on a scale running from from about 0.7. Electronegativity is measured on a variety of scales, the most common being the Pauling scale. Perhaps the most popular method of determining electronegativity is through Linus Pauling (1901-1995)'s model. The concept of electronegativity was put on a quantitative footing in 1932 by Linus Pauling in The Nature of the Chemical Bond. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. Linus Carl Pauling is the only person to have received two unshared Nobel Prizes - first in 1954 in chemistry. Besides being the greatest architect of chemistry, Pauling was a founder of molecular biology and a pioneer in quantum mechanics. Pauling combined chemistry and physics to solve various puzzles related to the nature of chemical bond. As one of his biographers has written Pauling's understanding of the chemical bond and molecular architecture is probably unsurpassed in the history of chemistry.
Location:
California, USA
ELECTRICAL CONDUCTIVITY PERIODIC TABLE
Periodic Table of Elements: Sorted by Electrical Conductivity. Electrical Conductivity for all the elements in the Periodic table. a table of relative electrical conductivity values; view a graph of relative electrical conductivity values; learn why relative electrical conductivity values. Why does electrical conductivity increase across the periodic table from sodium to magnesium to aluminium. Electrical resistivity and conductivity. Electrical conductivity is the measure of the amount of electrical current a ... Periodic Table - Interactive Periodic Table of the Elements. Electrical conductivity is a numeric description of a material's ability to carry. Conductivity is measured in Siemens, which are the reciprocal of electrical resistance. Metals have higher electrical conductivities than molecular elements. Periodic trends in boiling points and electrical conductivities.Periodic Properties Of Elements In The Periodic Table; Periodic Properties Of ... Electrical conductivity.
Labels:
conductivity,
electrical conductivity,
highest elctronegativity,
periodic table electronegativity
Location:
California, USA
Friday, September 28, 2012
IONIZATION ENERGY PERIODIC TABLE
Veiw a periodic table with first ionization energies. Chemical elements listed by ionization energy. The elements of the periodic table sorted by ionization energy. List of Periodic Table Elements Sorted by Atomic Number ..... Ionization energy The elements of the periodic table sorted by ionization energy The Periodic Table of the Elements (with Ionization Energies) This graph displays the periodic trend of ionization energy versus atomic number. Here's more information about what ionization energy is and what trends in ionization energy Ionization Energy - Ionization Energy of the Elements Thus the ionization energy of the elements decreases as you go down the periodic table because it is easier to remove the electrons. First ionisation energy - WebElements. IONISATION ENERGY. This page explains what first ionisation energy is, and then looks at the way it varies around the Periodic TableIonization Energy - Periodic Trends Why does the ionization energy increase as you go left to right down the periodic table what happens to the ionization energy What are the ionization energy trends seen in the periodic table The trend in effective nuclear charge accounts for the increase in ionization energy across a period.
Labels:
atomic radius,
electronegativity trends,
highest elctronegativity,
Iionization energy,
periodic table trends
Location:
California, USA
Saturday, September 22, 2012
IONIC RADIUS
Learn about the periodic table trends seen for the ionic radius of the elements. ATOMIC AND IONIC RADIUS. This page explains the various measures of atomic radius, and then looks at the way it varies around the Periodic Table. element for tables of the Effective Ionic Radii. The radii of atoms and ions can be defined and measured in a variety of ways for different purposes. Ionic radius: the nominal radius of the ions of an element in a specific ionization state. The Periodic Table of the Elements, showing relative atom sizes.Atomic and ionic radii are distances away from the nucleus or central atom that have different periodic trends. Periodic Trends — Atomic and Ionic Radii .... Unfortunately, it is not possible to determine the radius for every element on the periodic table in the same way. We can examine trends in ionic radii across a row of the periodic table by comparing data. the ionic radius increases as you go down. Ionic radii depend on Coordination Number. Atomic Radii Trends in the Periodic Table. The radii of anions are larger than the radii of ground state of the same element because an additional electron. Na+ has a smaller ionic radius than F. atomic radius decrese as you move across the periodic table. Periodic Table, showing for naturally occurring elements, their ionic radius. (10-8 cm, angstroms) for various oxidation states.
Location:
California, USA
Ionization Energy
Ionization energy (IE): The energy required to remove the outermost electron from an atom. Chemical elements listed by ionization energy. The elements of the periodic table sorted by ionization energy. First ionization energy (kJ/mol) the periodic trend of ionization energy versus atomic number. Thus the ionization energy of the elements decreases as you go down the periodic table because it is easier to remove the electrons. Using the periodic table to understand how difficult it is to ionize an atom. The 1st ionization energy of the element M is a measure of the energy. Ionization energy values are typically very high and follow trends throughout the periodic table. Ionization energy is the minimum energy required to remove an electron from the ground state of an atom. Ionization energy is a periodic trend. Ionization. Ionization is a process in which an electron is stripped off of a particle. If enough energy is available all the electrons on an atom can be removed. The first ionization energy varies in a predictable way across the periodic table. The ionization energy decreases from top to bottom in groups. The following periodic table shows the known first ionization energy data for the elements. We might expect the first ionization energy to become larger as we go across a row of the periodic table because the force of attraction between the nucleus.
HIGHEST ELECTRONEGATIVITY
The elements with the highest electronegativity are found in the upper right corner of the periodic table, excluding the noble gases.Electronegativity increases as you go from left to right across a period. Elements on the left of the period table have 1 -2 valence electrons. Electronegativity values generally increase from left to right within the Periodic Table of the elements. the relatively low-level quantities of bond lengths, electronegativities, and position in the periodic table. oxygen is more electronegative than chlorine. In the periodic table as we go to the right the size decreases so the electronegativity increases. Fluorine is the most electronegative element and caesium is the least electronegative atom. within metals electronegativity is high for metals at the top of the periodic table since they are small/dense and therfore have small radius. Electronegativity is how badly a element wants to a electron, F is the most electronegative element on the periodic table. periodic table, an atom's electronegativity is affected by both its atomic weight and the distance that its valence. Electronegativity generally increases as you move from left to right. This is because ionization energy steadily increases.
Thursday, September 20, 2012
Periodic Table Trends
Summary of Periodic Table Trends. Moving Left Right Atomic Radius Decreases Ionization Energy Increases Electronegativity Increases. Periodic table of electronegativity using the Pauling scale → Atomic radius ... molecule to attract electrons to itself. Electronegativity, metallic nature and atomic radius. ... Groups of the Periodic Table; Valence Electrons; Periodic Table Trends: Ionization Energy. Patterns of electronegativity in the Periodic Table. Electronegativity is a measure of an atom's "pull" on electrons. It is affected by the distance from the nucleus, electron-electron charge repulsion. Trends in Electronegativity in Periods of the Periodic Table In general, electronegativities of the elements in the same Period increases as you go from left to right. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7. If the atoms are equally electronegative, both have the same tendency to attract the bonding pair of electrons, and so it will be found on average half way between the two atoms. No electronegativity difference between two atoms leads to a pure non-polar covalent bond.A small electronegativity difference leads to a polar covalent bond.A large electronegativity difference leads to an ionic bond.
Wednesday, September 19, 2012
Electronegativity Trends
Periodic table of electronegativity using the Pauling scale. The tendency of an atom in a molecule to attract the shared pair of electron towards itself is called its electronegativity. What really powers a battery is the difference in electronegativity between the materials. Electronegativity values for each element can be found on certain periodic tables. the trends in electronegativity in the periodic table. Trends in Electronegativity in Periods of the Periodic Table. The ability of an atom in a molecule to attract shared electrons is called electronegativity. The higher the electronegativity of an atom, the greater its ability to attract shared electrons. The electronegativity of atoms decreases as you move from top to bottom down a group in the periodic table. GROUP TRENDS: Within a group of the periodic table, electronegativity decreases from top to bottom. Electronegativity is a measure of the attraction an atom involved in a bond .
Location:
California, USA
Subscribe to:
Posts (Atom)